Industries Served
Biotechnology & Biologics
Biotechnology & Biologics
Biotechnology & Biologics

Specialized expertise in human plasma derivatives, blood-derived therapies, and pathogen inactivation. Deep experience managing Biological License Applications (BLA) and pre-IND/IND pathways.
In Vitro Diagnostics
Biotechnology & Biologics
Biotechnology & Biologics

Experience with Point-of-Care (POC) devices, hemostasis management systems, and high-volume reagent manufacturing. Managed instrument and consumable portfolios.
Medical Devices (MedTech)
Biotechnology & Biologics
Medical Devices (MedTech)
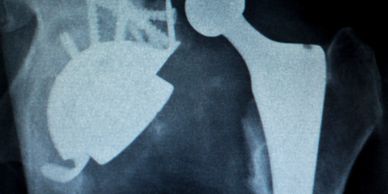
Extensive background in Class II and Class III devices, including surgical sports medicine (orthopedics), soft tissue implants, and disposable medical systems.
Sterile & Aseptic Mfg
Contract Manufacturing (CDMO)
Medical Devices (MedTech)

Validation of Class 10,000 (ISO 7) clean rooms, aseptic fill/finish, and sterilization modalities including Ethylene Oxide (EtO) and Gamma irradiation.
Contract Manufacturing (CDMO)
Contract Manufacturing (CDMO)
Contract Manufacturing (CDMO)

Deep operational experience on both sides of the table—serving as the client selecting/managing CDMOs for virtual biotechs, and as operational leadership within manufacturing orgs.
Industrial Manufacturing
Contract Manufacturing (CDMO)
Contract Manufacturing (CDMO)

Foundational engineering experience in high-volume industrial packaging and printing, providing a strong basis in automation, P&L management, and capital projects.
This website uses cookies.
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.